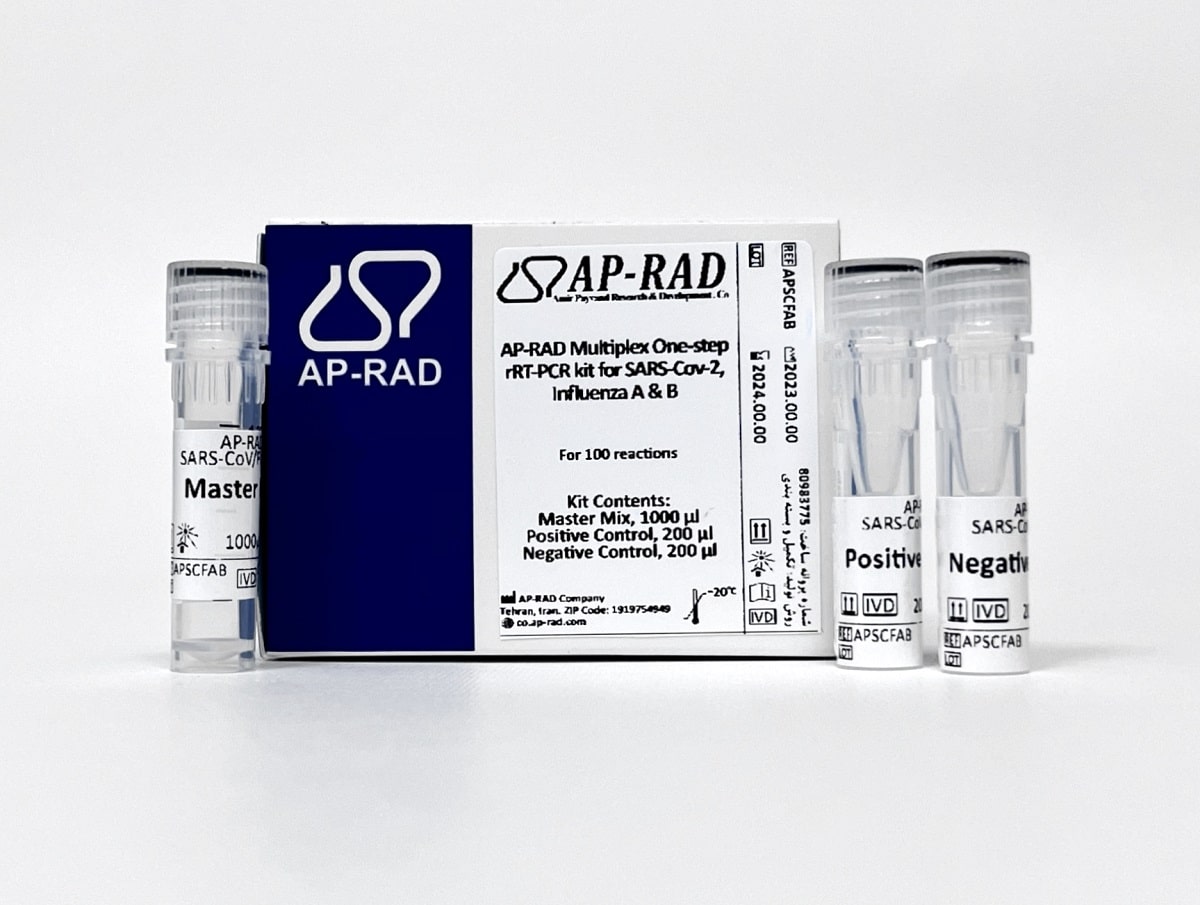
Description
Advantages and applications:
- Officially licensed by the General Directorate of Medical Equipment of the Ministry of Health and Medical Education (IVD)
- Evaluation of 3 specific genes:
- N gene for SARS-CoV-2 detection, with detection sensitivity of 0.4Copy / µL
- M gene for the diagnosis of influenza A, with a detection sensitivity of 1.0Copy / µL
- NS1 gene for the diagnosis of influenza B, with a detection sensitivity of 1.7Copy / µL
- Has internal control with CY5 label
- Single-step without the need for cDNA synthesis
- No need to prepare reagents
- Optimal stability of the kit