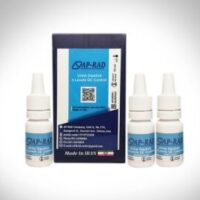
ISO 13485 certificate obtaning for AP-RAD products
No Comments
ISO 13485 certification leads to assurance to customers regarding safer production, the effectiveness of the quality management system of medical equipment and the fulfillment of legal requirements. The ISO 13485 standard in 2016 has replaced the previous edition standard ISO 13485:2003. Considering the importance of implementing ISO 13485 in ensuring…