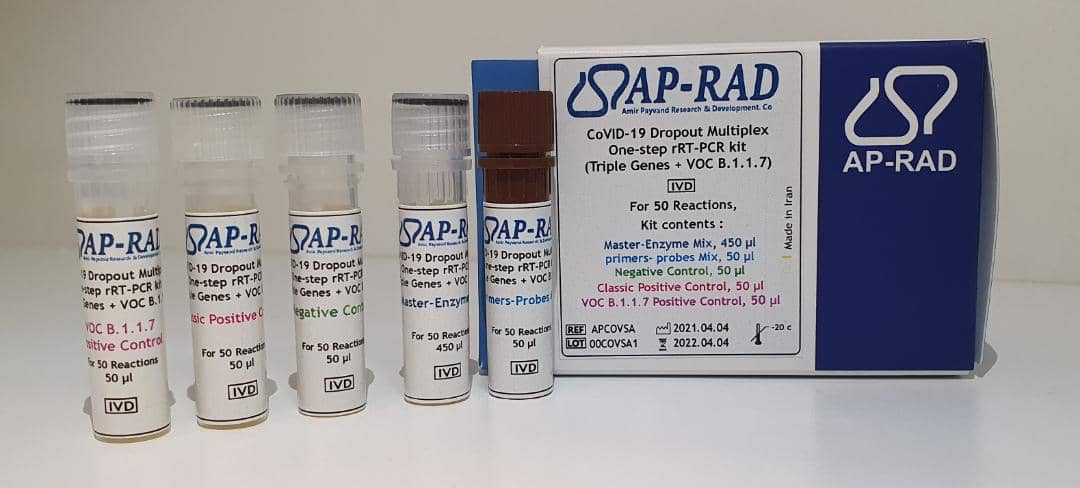
Description
Advantages and applications:
- Officially licensed by the General Directorate of Medical Equipment of the Ministry of Health and Medical Education (IVD)
- Evaluation of 3 viral genes N (nucleocapsid protein gene), ORF 1b, and S gene (Spike)
- Has internal control (RNase P) with CY5 label
- Classic CoVID-19 factor detection with research application for detection of B.1.1.7 or alpha variant (according to WHO classification)
- Detection Limit 0.2 copy / µL
- One-step without the need for separate cDNA synthesis
- Optimal stability of the kit