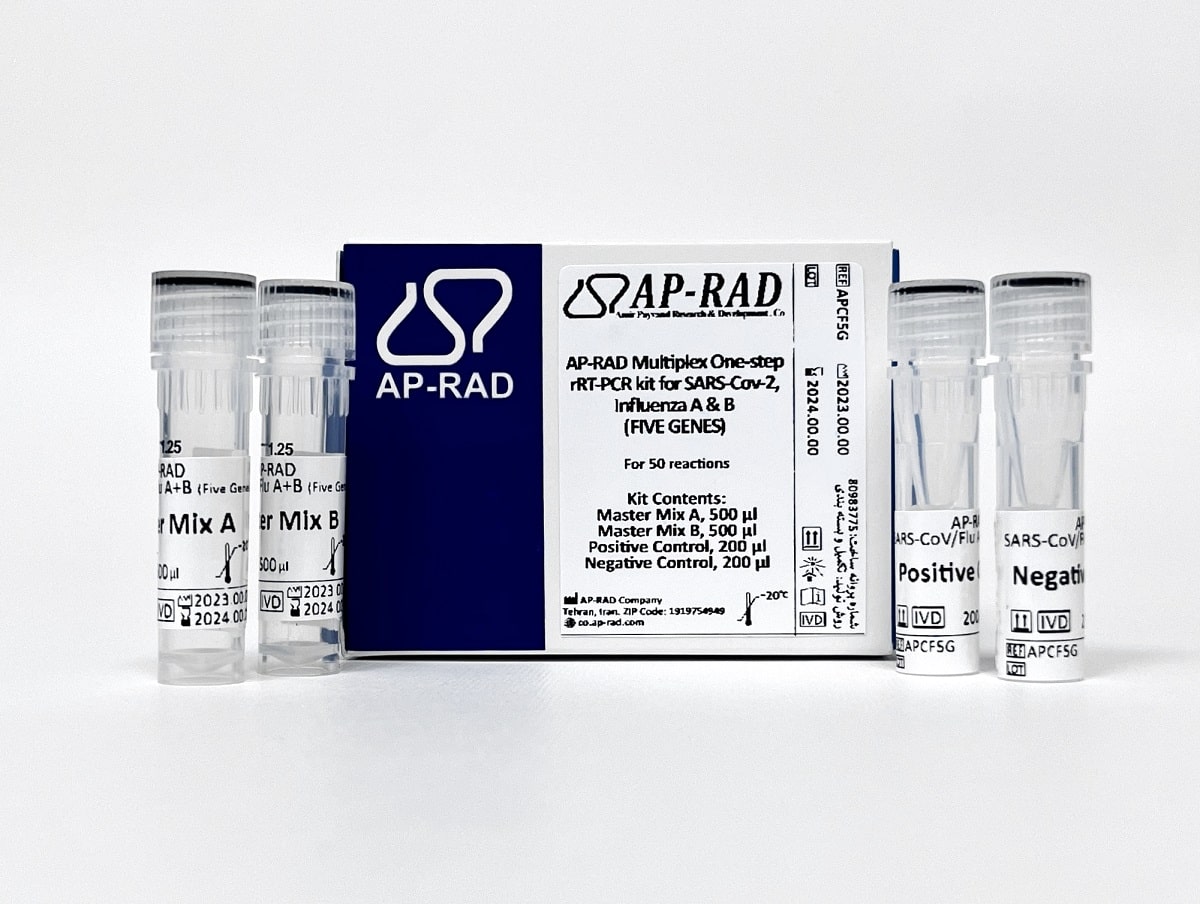
Description
Advantages and applications:
- Officially licensed by the General Directorate of Medical Equipment of the Ministry of Health and Medical Education (IVD)
- The only simultaneous detection kit for three-channel devices available in Iran, such as ABI Step One Plus
- Evaluation of 4 specific genes:
- N gene for SARS-CoV-2 detection, M gene for the diagnosis of influenza A
- Orf1b gene for SARS-CoV-2 detection, NS1 gene for the diagnosis of influenza B
- Use of RNase P gene as internal control on ROX channel
- Can be used on devices with green (FAM), yellow (HEX, VIC or JOE) and orange (ROX) channels
- Single-step without the need for cDNA synthesis
- No need to prepare reagents
- Optimal stability of the kit